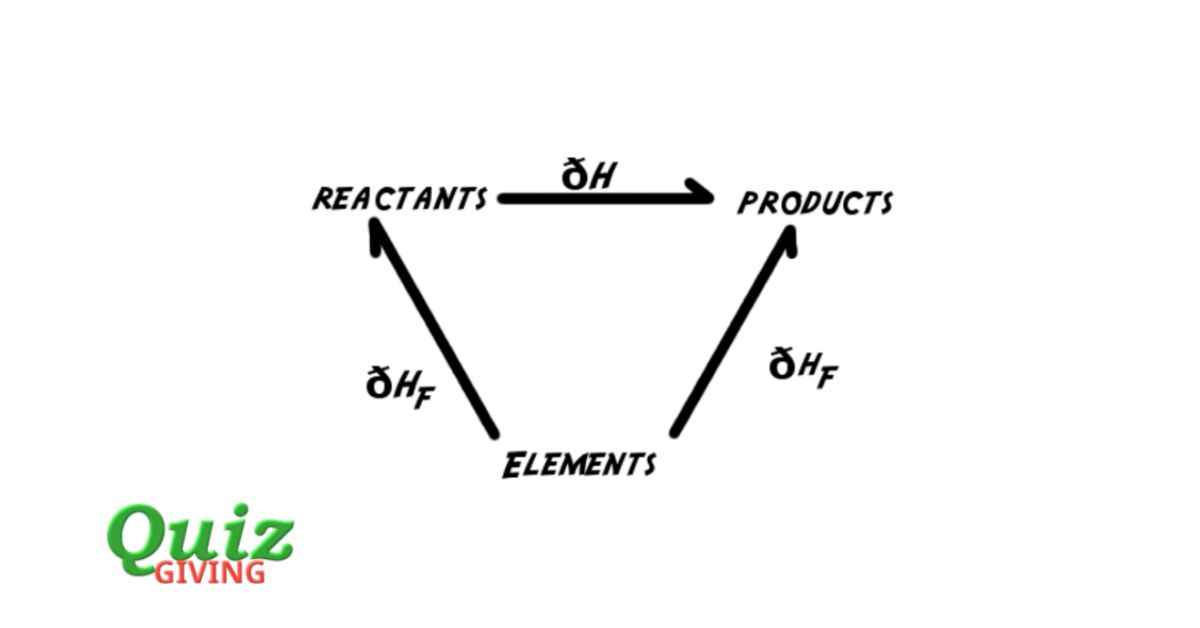
Are you ready to put your chemistry knowledge to the test? Get ready to unlock the mysteries of Hess’s Law with our Chemistry Conundrum Challenge! Hess’s Law is a fundamental concept in thermodynamics that allows us to calculate the enthalpy change of a chemical reaction. But don’t worry if you’re not familiar with it yet, because this quiz is designed to educate and entertain. In this quiz, you’ll be presented with a series of questions that will challenge your understanding of Hess’s Law and its applications. From calculating enthalpy changes to understanding reaction pathways, this quiz covers it all. So, whether you’re a chemistry enthusiast or a student looking to brush up on your knowledge, this quiz is perfect for you. Take the challenge and see if you can unlock the mysteries of Hess’s Law!